Back The fastest vaccine yet
Services
The fastest vaccine yet
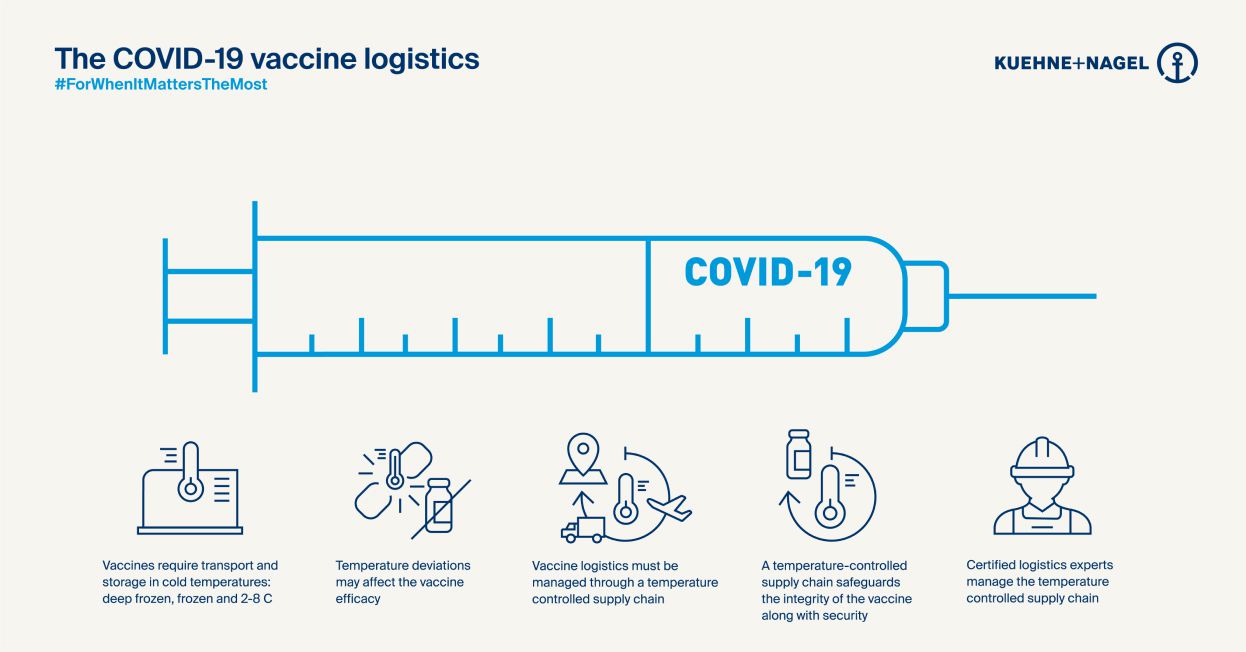
That means elements that would normally evolve in parallel with approvals – development of stable fillers, specialist packaging, clinical infrastructure and so on – will have to develop extremely quickly, too. Some of the new vaccines need to be kept at minus-70 degrees celsius. And a single shipment for a government customer could be 10 million doses – perhaps 60 or 70 pallets. So capacity, not just cooling, requires a massive effort.
“Talk now, talk often”
In a large-scale, multi-agency program, clarity in supply chains makes a world of difference. That’s why we need to think of new ways to frame the requirements at every stage of the process.
At Kuehne+Nagel, we have over a decade’s experience of specialist logistics with the pharma industry, so we know there are good reasons companies tend to be circumspect about forecasts and products – not least regulations. And messaging from governments has to be tightly controlled during a pandemic, too.
But to deliver the creative solutions that are needed imminently for a holistic response to Covid-19, those barriers have to drop. We know early predictions will be off – about volumes, precise climate control requirements or latent infrastructure available in the field. We know sometimes the messaging might be uncomfortable or sensitive.
To get quick solutions, that first and foremost are safe, but also ‘good enough’, we need as much information as possible, as soon as possible.
“For when it matters the most”
Over the last decade, meeting the needs of the global pharma industry changed the mindset at Kuehne+Nagel. For example, we quickly learned to apply our pharma quality management systems universally – just like the pharma industry itself. It demands an integrated approach between logistics’ elements – air, sea, road and warehousing – that we can deliver.
And just as the sector has put the patient at the heart of their businesses, our mission is clear: ‘for when it matters the most’. Our people know the work they do, their innovations, and the speed with which they deliver them, has a life-changing impact on millions.
We combine this mindset with our certification in global industry practices across different dimensions – from manufacturing to distribution to storage – the ‘GxP’ standards. That’s going to be incredibly important as the Covid-19 logistics effort moves from personal protective equipment (PPE – which is simple to store and transport) to more sensitive compounds.
“We will find a way”
We’re certain we can find innovative ways to get vaccines intact to distribution centers in record time. The project’s success depends on everything clicking together seamlessly and at pace. Manufacturers can make doses available. We can ensure they are where, and when, they need to be. And there is a crucial service side that needs to be in place too, to make sure that patients are informed, locations set up and much more.
Maintain momentum
How does this response retain momentum when the situation is evolving every day? There are three critical steps.
- Focus on the patient. Start with how the vaccine will get into people – the clinics, the ‘last mile’ channels, the local infrastructure – and work backwards to design co-ordination efforts. The many parties needed to make this happen will need to work together seamlessly with the patient as their shared mission.
- Create open channels of communication. We can’t restrict ourselves to talking only when data has been double-checked, or products finalised. We all need to adapt as we go. When so many parties must work in tight co-ordination to deliver at speed, they must all share infomation on their aspect of the supply chain.
- Pre-shipment solutions. Lots of providers want to play their part. But proven capability and experience are critical to the success of this program. Shipping vaccines has to be done by organizations that have been investing in this area and have the expertise to make it happen.